Abstract
Introduction: Hematological toxicity and infectious complications are both common and substantially contribute to non-relapse mortality of CAR T-cell therapy. The recently developed CAR-HEMATOTOX (HT) score represents a validated risk-stratification system of hematotoxicity, infections, and clinical outcomes for R/R LBCL (Rejeski et al. Blood 2021/JITC 2022). The score integrates parameters of pre-CAR-T hematopoietic reserve (e.g. ANC, hemoglobin, platelet count) and inflammation (e.g. CRP, ferritin). Whether the HT score is of diagnostic utility in relapsed/refractory multiple myeloma (R/R MM) remains unclear.
Methods: In this multicenter retrospective study, we characterized the influence of the HT score on toxicity and clinical outcomes in 102 patients receiving BCMA CAR-T with ciltacabtagene autoleucel (n=7) or idecabtagene vicleucel (n=95) in a standard-of-care setting across five international CAR-T centers. CRS and ICANS were graded according to ASTCT criteria. Neutrophil recovery phenotypes were defined as quick recovery vs. intermittent recovery vs. aplastic as previously described (Rejeski et al. Blood 2021). Early infection events (day 0-90) were defined as bacterial, viral or fungal based on microbiologic data or as a clinical syndrome of infection (e.g. pneumonia, cellulitis, cystitis) based on retrospective chart review. Severe (grade ≥3) infections were defined as requiring intravenous anti-infective agents and/or hospitalization. Kaplan-Meier estimates of progression-free (PFS) and overall survival (OS) were compared using the log-rank test.
Results: The baseline patient features were representative of R/R MM patients receiving BCMA CAR-T in a real-world setting: median age was 65 (range 39-81), median ECOG was 1 (interquartile range 0-1), median LDH was 200 U/L (IQR 173-240 U/L), and patients had received a median of 6 prior treatment lines (IQR 5-7). A total of 28% had high-risk genetic abnormalities (del17p, t(4;14), t(14;16)). Notably, the large majority of patients had received a prior autologous stem cell transplant (88.2%). On the last bone marrow (BM) assessment prior to CAR-T, greater than 5% clonal plasma cells were detected in 52% of patients. In terms of toxicity, the rate of severe (grade ≥3) CRS and ICANS was 4% and 8%, respectively. Tocilizumab was applied in 79/102 patients (77%); glucocorticoids were used in 43/102 patients (42%).
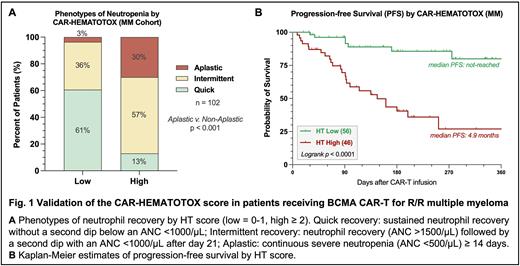
The median CAR-HEMATOTOX score was 1 (IQR 1-3), including 46 HThigh and 56 HTlow patients. When comparing the risk groups, we found that HThigh patients more frequently exhibited BM plasma cell infiltration (59% vs. 34%, p=0.05). Serum LDH levels did not significantly differ by HT score (median 215 vs. 193 U/L, n.s.). The duration of severe neutropenia (ANC<500/µL) was significantly increased in HThigh patients compared to their HTlow counterparts (9 vs. 3 days, p<0.0001). The proportion of patients displaying aplastic neutrophil recovery was markedly increased in the HThigh group (Fig. 1A: 30 vs. 3%, p<0.001).
The rate of severe CRS (9% vs. 0%, p=0.038) and severe ICANS (17% vs. 0%, p=0.001) was higher in HThigh patients, likely resulting in the increased use of glucocorticoids (57% vs. 30%, p=0.009). When studying infectious complications, we found that infections of any-grade were more common in the HThigh cohort (61% vs. 25%, p=0.0003). This was particularly evident for severe infections (39% vs. 3.6%, p<0.0001), including 2 fatal bacterial infections in HThigh patients. Overall, the increased toxicity burden observed in the HThigh cohort translated into a longer median duration of hospitalization (12 vs. 8 days, p<0.0001). Finally, a high HT score was associated with inferior PFS (Fig. 1B: 1-year PFS 80% vs. 27%, p<0.0001) and OS (1-yr OS 93% vs. 62%, p<0.0001).
Conclusions: These findings emphasize the importance of baseline inflammatory state and hematopoietic function for the subsequent development of toxicity and early progression in patients receiving BCMA-directed CAR-T. The easy-to-use CAR-HEMATOTOX score enables early risk-stratification prior to lymphodepletion. Considering their overall low risk of severe toxicity, HTlow patients represent an attractive population to explore outpatient CAR-T application. On the other hand, patients with a high HT score likely benefit from intensified monitoring, anti-infective prophylaxis, early G-CSF, and awareness for a potential stem cell boost.
Disclosures
Rejeski:Novartis: Honoraria; Kite/Gilead: Other: Travel Support, Research Funding. Hansen:BMS IMW Ide-Cel Academic Advisory Board: Membership on an entity's Board of Directors or advisory committees; OncLive: Honoraria; Survivorship: Honoraria. Sesques:Chugai, Novartis, and Kite/Gilead: Research Funding. Freeman:Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria; Janssen: Honoraria, Research Funding; Amgen: Honoraria; Incyte: Honoraria. Alsina:BMS: Research Funding; BMS, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Theurich:Takeda: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; GSK: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Locke:Society for Immunotherapy of Cancer: Other: Education or editorial activity; Imedex: Other: Education or editorial activity; Clinical Care Options Oncology: Other: Education or editorial activity; CAREducation: Other: Education or editorial activity; BioPharm Communications: Other: Education or editorial activity; ASH: Other: Education or editorial activity; Leukemia and Lymphoma Society: Research Funding; Aptitude Health: Other: Education or editorial activity; ), National Cancer Institute: Research Funding; CERo Therapeutics: Research Funding; Takeda: Consultancy; Sana: Consultancy; Daiichi Sankyo: Consultancy; BMS: Research Funding; A2: Consultancy; Celgene: Consultancy; Other: Patents & Royalties: patents, royalties, other intellectual property from several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy.; Wugen: Consultancy; Umoja: Consultancy; Novartis: Consultancy, Research Funding; Legend Biotech: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Janssen: Consultancy; Iovance: Consultancy; GammaDelta Therapeutics: Consultancy; Emerging Therapy Solutions Gerson Lehrman Group: Consultancy; EcoR1: Consultancy; Cowen: Consultancy; Calibr: Consultancy; Cellular Biomedicine Group: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Bluebird Bio: Consultancy, Research Funding; Allogene: Consultancy, Research Funding; Amgen: Consultancy. Bachy:Kite, Gilead, Novartis, Roche, Incyte, Miltenyi Biotech, Takeda, Sanofi: Honoraria; Roche, Gilead, ADC Therapeutics, Takeda, Novartis, Incyte: Membership on an entity's Board of Directors or advisory committees; Amgen, BMS: Research Funding; Hospices Civils de Lyon: Current Employment. Jain:Incyte: Research Funding; MyeloidTx: Consultancy; BMS: Consultancy; Novartis: Consultancy; Kite Pharma: Consultancy, Research Funding. Lin:Bluebird Bio: Consultancy, Research Funding; Sorrento: Consultancy; Vineti: Consultancy; Celgene: Consultancy, Research Funding; Gamida Cell: Consultancy; Merck: Research Funding; Takeda: Research Funding; Janssen: Consultancy, Research Funding; Novartis: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Legend: Consultancy; Juno: Consultancy. Subklewe:Roche: Consultancy, Research Funding; Seagen: Research Funding; Morphosys: Research Funding; Seattle Genetics: Research Funding; Bristol-Myers Squibb: Research Funding; Janssen: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Takeda: Other: Travel support; Miltenyi Biotech: Research Funding; Pfizer: Consultancy; Gilead: Consultancy, Research Funding, Speakers Bureau; Celgene/BMS: Consultancy, Speakers Bureau; Amgen: Consultancy, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal